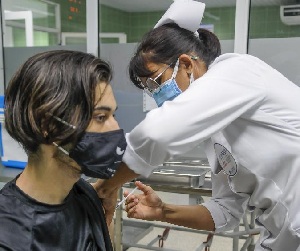
 With the vaccination of the first 25 volunteers in the 12-18 age group, Phase I clinical trials in pediatric ages of the Soberana 02 candidate anti-COVID vaccine developed by the Finlay Vaccine Institute (IFV) began yesterday, June 14.
With the vaccination of the first 25 volunteers in the 12-18 age group, Phase I clinical trials in pediatric ages of the Soberana 02 candidate anti-COVID vaccine developed by the Finlay Vaccine Institute (IFV) began yesterday, June 14.
As anticipated, the process was initiated at the Juan Manuel Márquez Pediatric Hospital in Havana, strictly adhering to protocols, with all the rigor and organization required, beginning the first day.
Dr. Meiby de la Caridad Rodríguez González, director of Clinical Research at the IFV, explained that the first volunteers were among those who responded to a call on June 11, when their parents expressed interest in having their children participate in the trial.
She noted, “We received more volunteers than were included in the study, who, after the rigorous process required for Phase I, 25 were selected, which was the sample size determined for the 12 to 18 age group.”
Once the parents and adolescents agreed to take part in the study, signing the informed consent and assent document, the clinical evaluation process began and they were summoned to the hospital Monday.
(Taken from Granma)
Prior to the injection, another physical evaluation and verification of vital parameters were again performed, to make a final decision about their inclusion and proceed to inoculation of the candidate vaccine.
Subsequently, as has been the case with the adult population, the young volunteers remained under observation for one hour, to address and record any possible adverse side effects of the immunogen.
In this regard, Dr. Rodriguez Gonzalez pointed out that, in the event of any serious reactions, all conditions are prepared at the clinical site within the hospital which has extensive experience in conducting trials in pediatric age groups.
After the hour of observation, specialized health personnel re-checked subjects’ vital signs, as well as the condition of the vaccination site, before discharging the volunteer and making follow-up appointments for the following day, as well as within 48 and 72 hours.
“We are optimistic that we are not going to see serious adverse side effects, the trial has been rigorously prepared, we chose this site, with all the conditions in place, so the research team, parents and everyone in general can be sure that the clinical trial will be carried out successfully,” Dr. Rodriguez insisted.