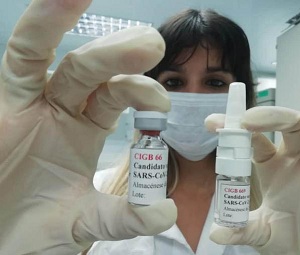
 After a careful review of the information presented, including evidence from pharmaceutical development and studies in animals, the Center for the State Control of Drugs, Equipment and Medical Devices (Cecmed), authorized the clinical trials of two new Cuban candidate vaccines to prevent COVID-19, given the names of Mambisa (CIGB-669) and Abdala (CIGB-66).
After a careful review of the information presented, including evidence from pharmaceutical development and studies in animals, the Center for the State Control of Drugs, Equipment and Medical Devices (Cecmed), authorized the clinical trials of two new Cuban candidate vaccines to prevent COVID-19, given the names of Mambisa (CIGB-669) and Abdala (CIGB-66).
Trials of the first, created by researchers at the Genetic Engineering and Biotechnology Center (CIGB), affiliated with the BioCubaFarma Enterprise Group, and administered nasally, will be conducted in Havana, while the human studies of Abdala, administered intramuscularly, will take place in the city of Santiago de Cuba.
Dr. Eulogio Pimentel Vázquez, CIGB general director, told Granma that, in both cases, the objective is to determine, first of all, the safety of the candidate vaccines in healthy adults between 18 and 54 years of age, and secondly, the vaccination’s ability to induce the production of antibodies that promote the appropriate bodily response to the SARS-COV-2 virus, which causes COVID-19.
He noted that the effectiveness of the two administration methods and the combination of these will also be explored during the trials.
After receiving approval from the national regulatory authority, the recruitment of volunteers to participate in the study began immediately.
According to one of the most visited sites devoted to progress in COVID-19 vaccine efforts around the world (https://www.covid-19vaccinetracker.org/), as of November 24, 237 projects had been registered, 40 of which have reached the clinical trials stage.
Cuba’s internationally recognized pharmaceutical and biotechnological industry has been involved in the race to develop vaccines against COVID-19 since March, this year, when an accelerated program was launched.
Toward this end, several different candidate vaccine projects are underway, based on a variety of industrial technologies, means of administration and types of antigens.
These projects do not compete for human resources or industrial capacity. They complement each other and make it possible to expedite the process, to reach the goal of vaccinating the entire population, as well as others in need.
Our scientists have developed four candidate vaccines thus far: Soberana 1 and 2, Mambisa and Abdala.
According to the aforementioned web site, there are currently no other vaccine candidates in the clinical stage using the nasal route for administration (hence the novelty of Mambisa), although there is a marked interest and need to do so, Dr. Pimentel indicated.
(Taken from Granma)